In 1944, during the Second World War, the Curtin Labor government introduced legislation for the Pharmaceutical Benefits Scheme (PBS). With a national need for medicines including penicillin, streptomycin and sulphonamides, the bill allowed any Australian resident to access certain medicines at no charge after presenting a doctor’s prescription to their pharmacist.
Although the legislation passed, it was defeated in the High Court in 1945 on constitutional grounds, leading to the subsequent Social Services referendum in 1946, which gave the Parliament the power to make laws with respect to pharmaceutical benefits. The PBS continued to expand through the late 1940s and 1950s, culminating in a comprehensive scheme by 1960 (through a 1959 amendment to the National Health Act 1953). With this, the PBS became a comprehensive scheme, no longer limited to a small number of expensive and lifesaving drugs.
The PBS remains a cornerstone of Australia’s universal healthcare system, dispensing 226 million subsidised prescriptions across the 2024–25 financial year.
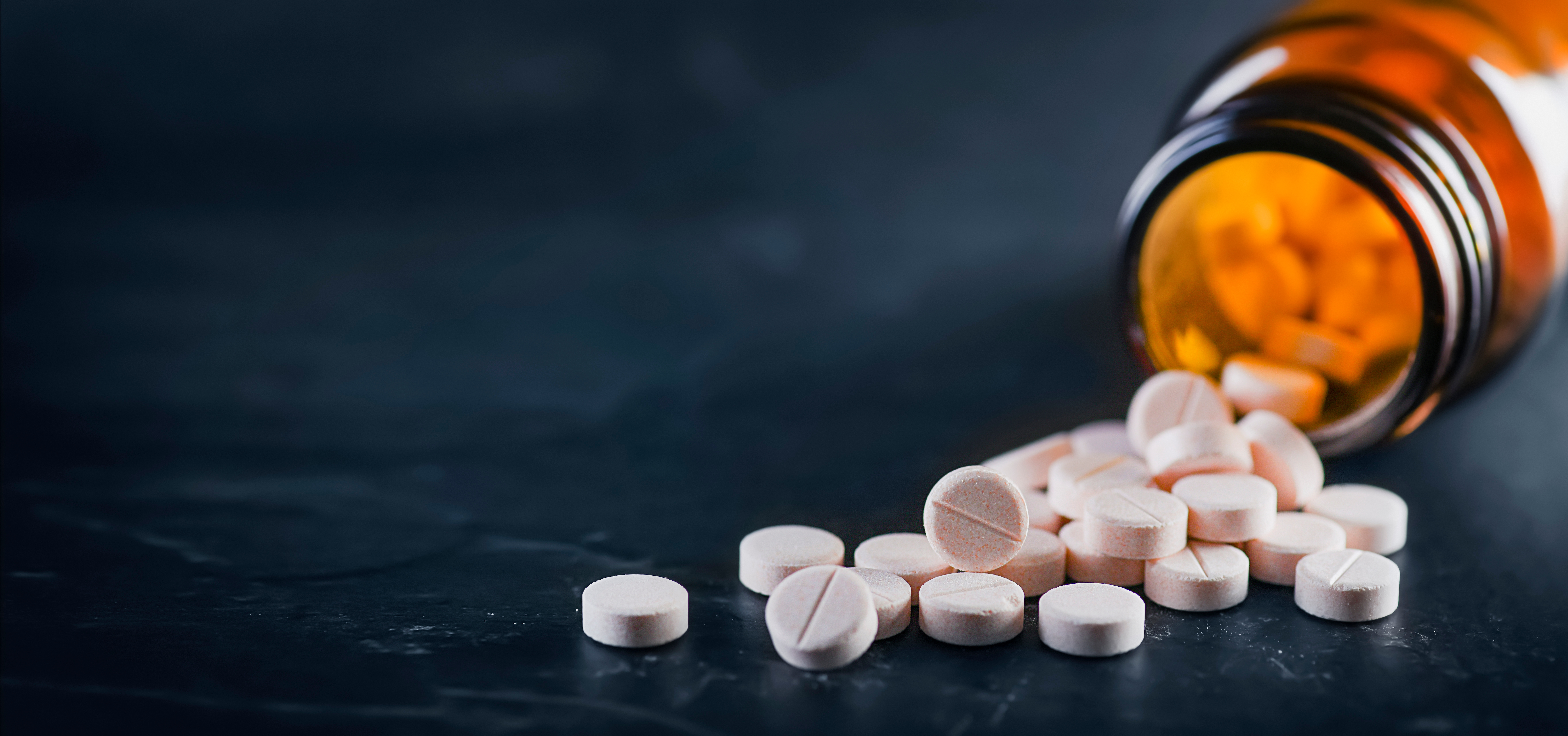
As Australia deals with uncertainty in the global pharmaceutical market and increasing listing pressure, join Nexus APAC as we examine medicine shortages, and explore what they mean for patients, practitioners, and the future of Australia’s healthcare landscape.
The Therapeutic Goods Administration (TGA) legally defines a medicine shortage as occurring “at a particular time if at any time in the 6 months after that particular time, the supply of that medicine in Australia will not, or will not be likely to, meet the demand for the medicine for all of the patients in Australia who take, or who may need to take, the medicine.”
At present, over 380 medicines are in shortage in Australia, according to the TGA Medicine Shortages Information Initiative. Such situations arise from a complex interplay of global and systemic factors that influence Australia’s capacity to manage shortages. As a relatively small player, Australia accounts for around 2 percent of the global pharmaceutical market and imports over 90 percent of prescription medicines. As such, global disruptions often leave Australia exposed as manufacturers and suppliers prioritise larger markets.
Beyond global supply constraints, regulatory hurdles can exacerbate medicine shortages. Medicines suppliers must navigate several layers of approvals in Australia, including TGA registration and price negotiations. The PBS listing process is rigorous, requiring comprehensive clinical and economic evaluations by the Pharmaceutical Benefits Advisory Committee (PBAC) before a medicine can be subsidised. This approach is designed to ensure value for taxpayers and equitable access, but can delay the entry of new suppliers, alternative brands, or substitutes during periods of shortage.
In addition, under the Government’s price disclosure policy, sponsors of low margin generic medicines are required to provide periodic data to the Commonwealth which informs the pricing of those medicines. While important for cost containment from a government perspective, this approach place additional pressure on low margin products, potentially discouraging continued supply, reducing competition and heightening vulnerability to disruptions.
Medicine shortages have significant and cascading impacts across Australia’s healthcare system, affecting patients, frontline pharmacy services, and the sustainability of the PBS. Patient treatment continuity can be severely disrupted, forcing individuals to switch brands, alter dosing schedules, or temporarily cease therapy.
Under section 19A of the Therapeutic Goods Act 1989, the TGA can temporarily approve overseas registered medicines to address shortages, offering an effective stopgap measure. The increasing use of this measure indicates the impact of shortages in recent years, with section 19A approvals rising to a peak of 130 in 2024, up from 26 in 2021.
These disruptions are especially harmful for people managing chronic or complex conditions, where stability is essential to preventing deterioration. Shortages can heighten anxiety and erode confidence in the PBS as a reliable and affordable system of medicine supply, particularly when patients must contact multiple pharmacies to obtain essential medications. Clinician accounts and professional reporting highlight increased workloads, safety risks and higher out of pocket costs for some consumers during prolonged shortages.
Pharmacies carry a substantial operational burden during shortages, spending considerable time sourcing stock, coordinating with wholesalers, and advising patients on alternatives. This additional workload diverts attention from clinical services such as medication reviews, vaccinations, and chronic disease management. Some pharmacies also incur higher costs by holding additional inventory or purchasing more expensive substitutes. Rural and remote pharmacies, already limited in supply options, experience these pressures even more acutely.
At the request of industry, the Government completed a comprehensive Health Technology Assessment (HTA) Policy and Methods Review in September 2024, and established the HTA Review Implementation Advisory Group (IAG) to guide critical reforms in response to the recommendations of the Review.
The Review aimed to modernise Australia’s evaluation and reimbursement pathways for medicines and health technologies, with a strong focus on accelerating access without compromising safety or cost effectiveness. The gradual implementation of HTA Review recommendations is expected to ease pressure on the PBS and support faster listing of alternative brands and substitutes during shortages.
Ultimately, these reforms aim to strengthen supply resilience and ensure patients receive timely access to essential medicines. While Australia has long relied on a robust regulatory framework and global supply chains to safeguard medicine access, it is now well placed to leverage the outcomes of the HTA Review to build a more agile and resilient PBS capable of withstanding the pressures of an increasingly volatile pharmaceutical landscape.
Latest posts by Nexus APAC (see all)
- Examining Medicine Shortages in Australia - February 7, 2026
- The Secretaries of Federal Departments - January 31, 2026
- Merry Christmas from the Nexus APAC Team - December 19, 2025